Drugs that have already been approved for comparable diseases could make a quick and important contribution to the containment of a pandemic. Indeed, a number of clinical trials are testing the efficacy of known drugs against the corona virus (repurposing). However, while the number of COVID 19 studies is an impressive 8,311, to date, only 11 studies have been focused on repurposing (source: clinictrial.gov).
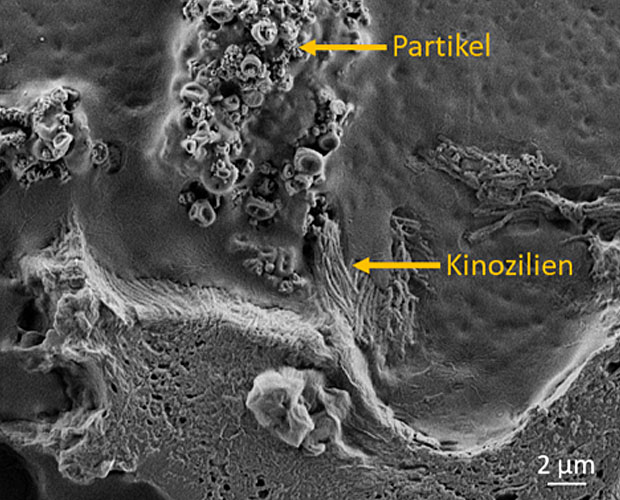
A large amount of effort is required to identify suitable drug candidates in advance (at the preclinical stages) and this presents a barrier to the wider implementation of this technique. Given this, in vitro test models (i.e., tissues cultivated in test tubes) can bring significant advantages. The use of human 3D or 2D tissue models of the conducting airways cultured at the medium-air interface and of alveolar lung tissue organoids offers the possibility of rapidly identifying suitable agents during the preclinical phase that could prevent viruses like SARS-CoV-2 replicating in human cells.
This is why the Translational Center for Regenerative Therapies (TLC-RT) and the associated Chair of Tissue Engineering and Regenerative Medicine (TERM) at the University of Würzburg have, within the scope of the interdisciplinary RoboCure Project, focused on exploring the use of flexible and interactive robotic technology in the production of in vitro organoid cell culture models of the respiratory tract. In the medium term, this process is to be carried out under stringent regulatory GMP conditions (medical devices: ISO 13485, pharmaceuticals: GMP (Good Manufacturing Practice)) taking into account relevant quality assurance criteria for individualized diagnostics and therapy.
Robot-assisted production enables rapid and standardized preclinical identification and qualification of substances that have already been approved for other applications (repurposing studies), thus shortening the route to immediate clinical translation.
By automating the complex manufacturing process of these human tissue models, the RoboCure project will not only enable time savings in the generation of these models but will also increase standardization and reproducibility. The project is based on preliminary work such as previously developed cell culture models and the automated system for skin tissue production. Moreover, a different project is already seeing organoid models for intestine models being produced at the facility. Significant barriers have been overcome, such as the handling of viscous media by means of a bioplotter. As a result, it can be assumed that, in the short term, this project is well placed to contribute to tackling the crisis. In the medium to long term, technological know-how will allow for a broadly applicable automated testing platform to be established.